Medical Biotech
Bio-Pharmaceutical is known as Red Biotechnology. This branch of biotechnology is related to health care. The main utilization of this branch is shown in the field of medicine. Red biotechnology helps to improve the quality of life.
Red-Biotechnology has a wide range of applications. Many applications are shown in medical and health science, genetic engineering, gene therapy, and many more fields related to the medical line.
20 Major Applications of Red Biotech
Red biotechnology includes sciences related to clinical research and trials that provide scientific analysis of the impact, risk, and benefits of medicines. The clinical research help in trial methodologies to designed materials.
Gene Therapy is the process of inserting genes into cells to treat disease. Gene therapy primarily involves genetic manipulations in animals or humans to correct disease and keep the organism in good health. So, red biotechnology shows advances in research that help to understand the genetic basis of inherited disease via gene therapy.
Genetic engineering involves the manipulation of genetic material (DNA). The base of genetic engineering is based upon the Recombinant technology i.e. rDNA technology, Gene cloning, Genetic modifications, and new genetics. All of these techniques are the grass root of genetic engineering.
Genetic engineering involves the manipulation of genetic material (DNA). The base of genetic engineering is based upon the Recombinant technology i.e. rDNA technology, Gene cloning, Genetic modifications, and new genetics. All of these techniques are the grass root of genetic engineering.
Recombinant DNA Technology is a boon to produce new generation vaccines. There are broadly categorized recombination vaccines into three groups- subunit recombinant vaccine, attenuated recombinant vaccines, and vector recombinant vaccines. There is a possibility of vaccinating several diseases with one recombinant vaccinia virus.
Red biotechnology plays a very important role in cancer research treatment. Biotechnology has to assume in the differential diagnosis of cancer, early detection of cancer, progression control, and proper treatment of cancer. Numerous techniques based on biotechnology field that is used in various cancer research program.
Stem cells are basically introduced into the damaged area of the body, with the help of stem cells we can replace the damaged area. Stem cells have the main area like bone marrow, transplants, replacement of damaged heart tissues, replacement of damaged nerve tissues. This is very helpful in our medical science.
There is various disease that is caused due to pathogens and inherited genetic defects. Identification and diagnosis of this disease based upon the DNA.ie. the genetic material of the living organisms. The presence of a disease-causing pathogen can be detected by identifying a gene or set of genes of the organism.
Biochips is a very important technique, that is the combination of biotechnology, electronics, and computers. Biochips is a collection of a miniaturized test site that is capable of performing thousands of simultaneous biochemical reactions. Biochips quickly screen a large number of biological analyses for the detection and extensive study of genomic, proteomic, and functional genomic analysis.
Pharmacogenomics is the study related to genome role in drug response that helps in research to show how a person's gene effects and persons respond to medications. This study helps to improve health by helping the doctor to find the medicine that will safe to take.
Tissue engineering is the creation of human tissue outside the body that is further used for later replacement. This is an also important technique in medical research.
Biopharmaceutical is a combination of biotechnology and pharmaceutical science. Components of living organisms, their extraction, and byproducts are used to prevent and treat the disease.
Medical biotechnology is widely used for the diagnosis, prevention, and treatment of various diseases. To treat bacterial infection antibiotic is used. By using biotechnology based techniques and methodologies we can improve the quality of medicine, and also discover new drugs for the further advanced treatment of disease and infection.
It is an interdisciplinary branch that is used to repair and regenerate the damaged cells and tissues and further restore their normal function. Regenerative medicine based on stem cell biotechnology.
Genetic testing is used to examine a sample of blood or other body fluids, and genetic markers that help to indicate the presence and absence of genetic disease. Genetic tests serve many purposes like identity testing, used to determine child potentiality, inherited disease, etc. Common test diagnostic tests under genetic testing are -ultrasonography, amniocentesis Quadruple screen, etc.
The molecular diagnostics technique is basically a collection of various techniques based on molecular biology that is applied to medical testing. These techniques are very helpful to diagnose and monitor the disease. These types of diagnostic techniques are very beneficial for the detection of a wide range of medical specialisms and genetic prediction of drugs.
Achievement of maximal expression of cloned genes can be achieved by manipulations. Gene expression is regulated by molecule influence through DNA transcription and RNA translation. The manipulation of gene expression is carried out in both prokaryotic and eukaryotic cells.
Monoclonal antibodies are a single type of antibody that is directed against a specific antigenic determinant. Monoclonal antibodies have a wide range of applications that can be applied in diagnostic applications, for therapeutics uses, protein purification, and shows it’s various miscellaneous applications in health care.
In biotechnology science, recombinant DNA technology can be fruitfully employed to produce human proteins that can be used for the treatment of genetically linked diseases.
A certain disorder like cancer, viral, parasitic infections, and inflammatory diseases result in the overproduction of certain normal proteins. To treat these types of diseases we need to blocking transcription by using a single-stranded nucleotide sequence that hybridizes with the specific gene.
Antisense therapy is used to the inhibition of both translation and transcription by blocking the transcription factor responsible for specific gene expression.
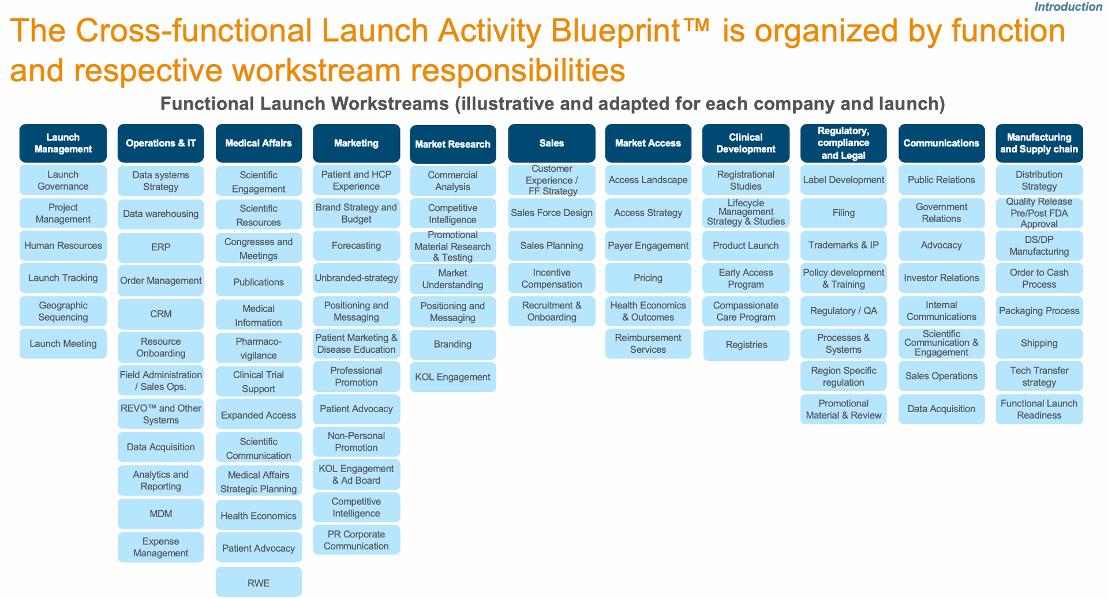
Red Biotech - Product to Market
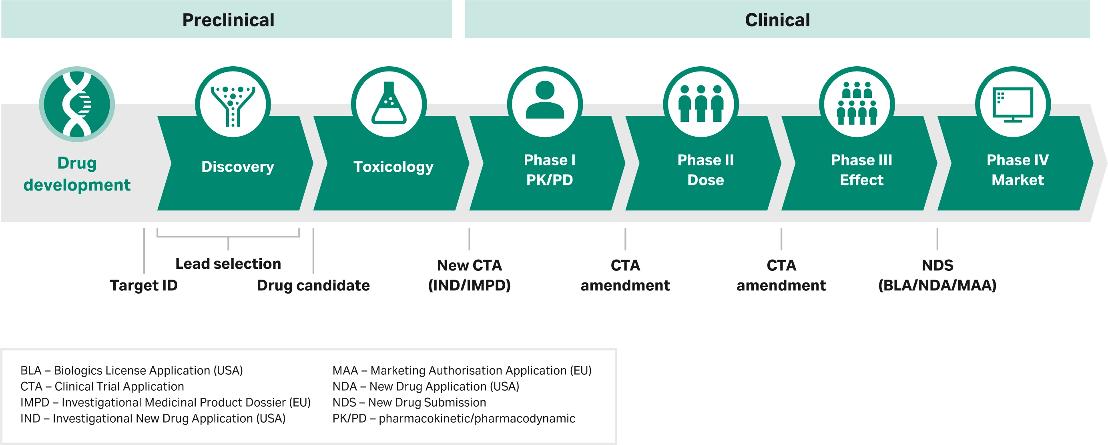
What is process development for biopharma ?
Biopharma process development comprises the activities that help you create a series of steps to produce a biomolecule – a monoclonal antibody (mAb), recombinant protein, viral vector, or other product that comes from a biological origin.
Bioprocess development is often divided into upstream process development and downstream process development. Those activities must be combined with the right analytics, so you can accurately measure what you’ve identified as your product’s critical quality attributes (CQAs) as you develop and refine your processes.
Process development activities will vary by your type of biomolecule, as well as the stage of the drug development process you are in – preclinical, early clinical (Phase I/Phase II), or late clinical (Phase III/Phase IV). Regulatory requirements will guide many of these activities.
At early stages of drug development, you will develop a process that is ‘good enough’ to meet the needs of that stage. However, it’s important to keep the end goal in mind. Ultimately, you will need a process that translates to a manufacturing environment, one that is easy to scale up throughout clinical trials and to the market. By the time you reach Phase III in the drug development process, ‘good enough’ is not sufficient. Instead, you will switch your focus to making sure you have a robust upstream or downstream process that delivers a high yield and high productivity. Also, your process must be cost-effective and reproducible.
Source:
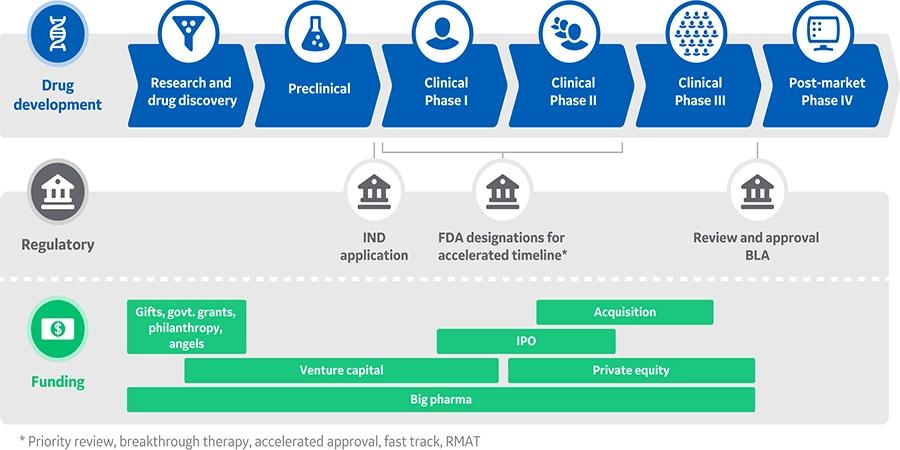
Overview of biopharmaceutical development and production in the United States.
Source:
https://www.cytivalifesciences.com/en/us/solutions/emerging-biotech/knowledge-center/funding-considerations-for-early-stage-biopharma
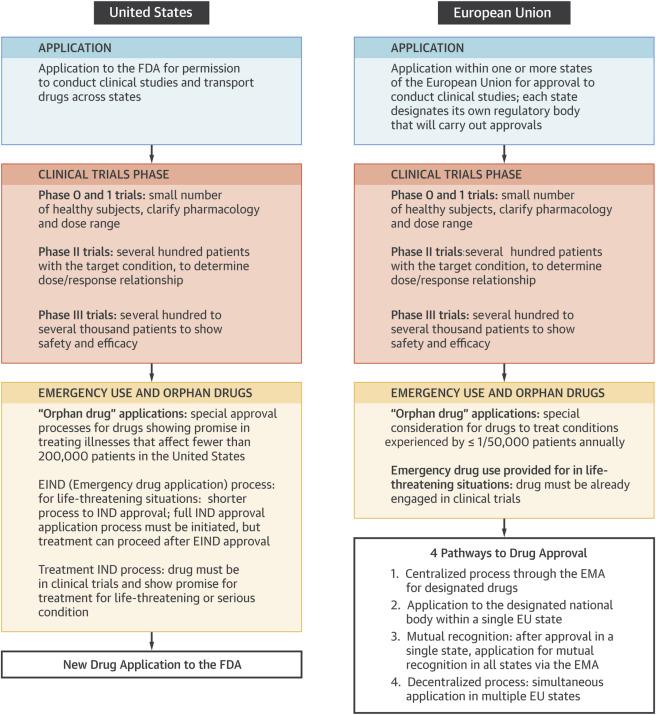
FDA vs EMA
New Drug Approval Process, United States vs European Union.
Source:
https://www.sciencedirect.com/science/article/pii/S2452302X16300638
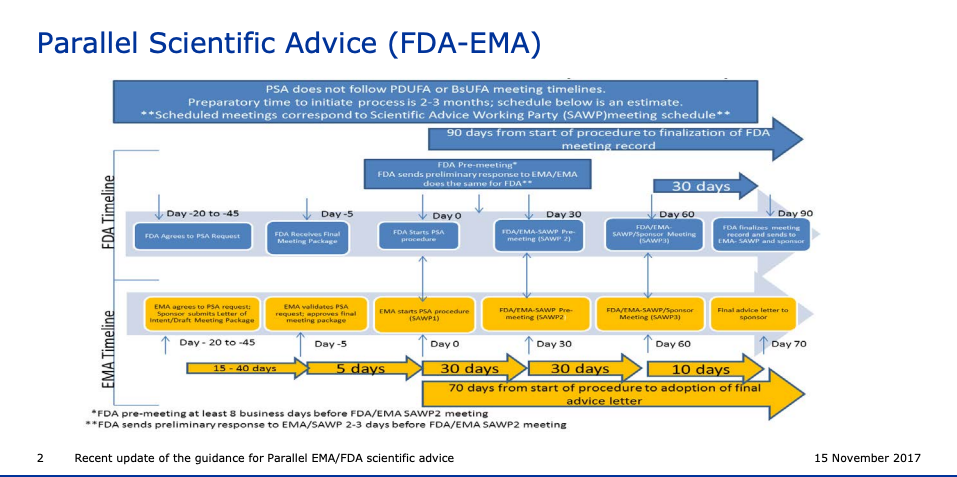
FDA vs EDA Timeline
The guidance for Parallel EMA/FDA scientific advice.
Source:
EUROPEAN PHARMACEUTICAL INCENTIVES FRAMEWORK

Feature One
With the number of new medicines approved every year, the system is working: it enables a pipeline of currently over 7000 medicines in development despite the high risk of failure.
Source:
https://www.efpia.eu/about-medicines/development-of-medicines/intellectual-property/
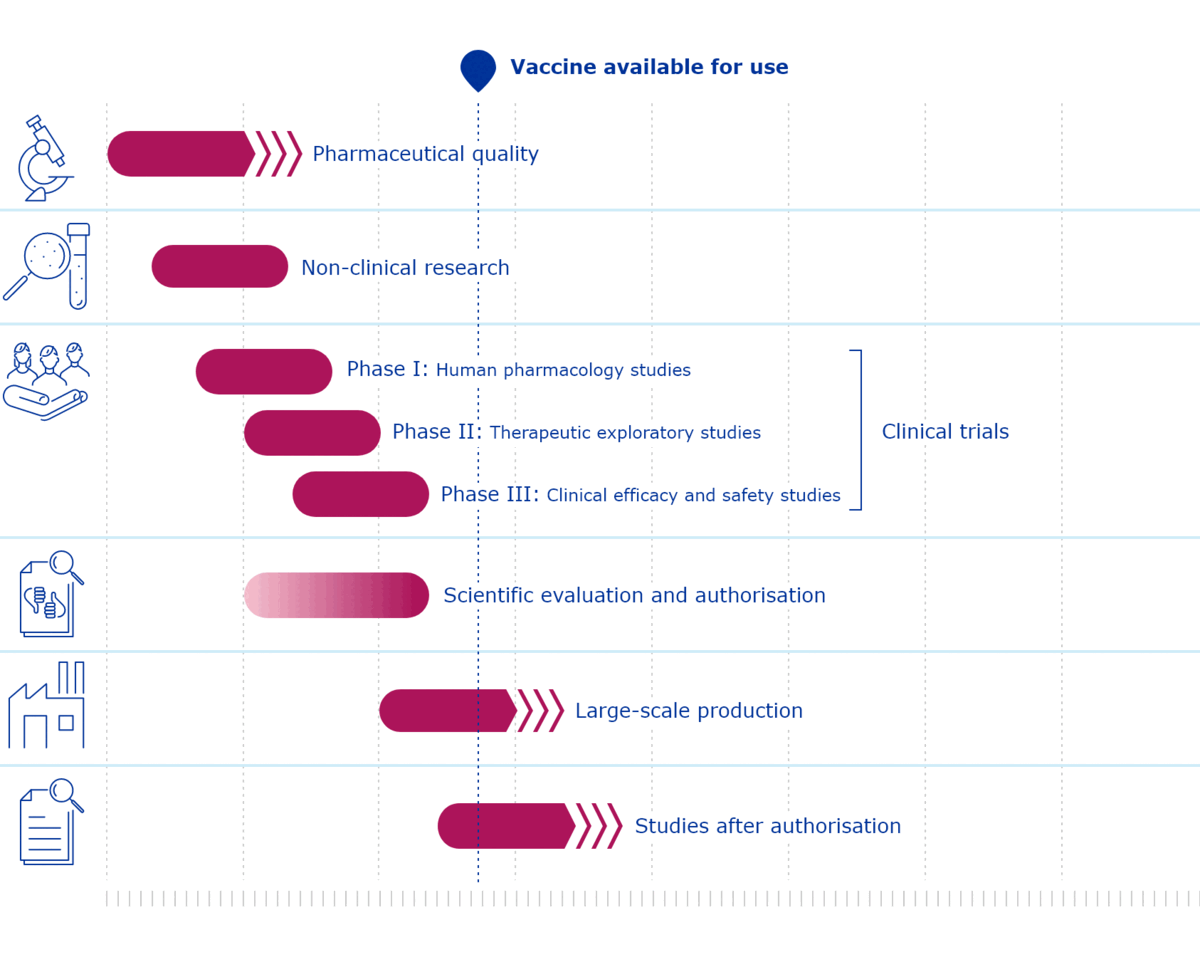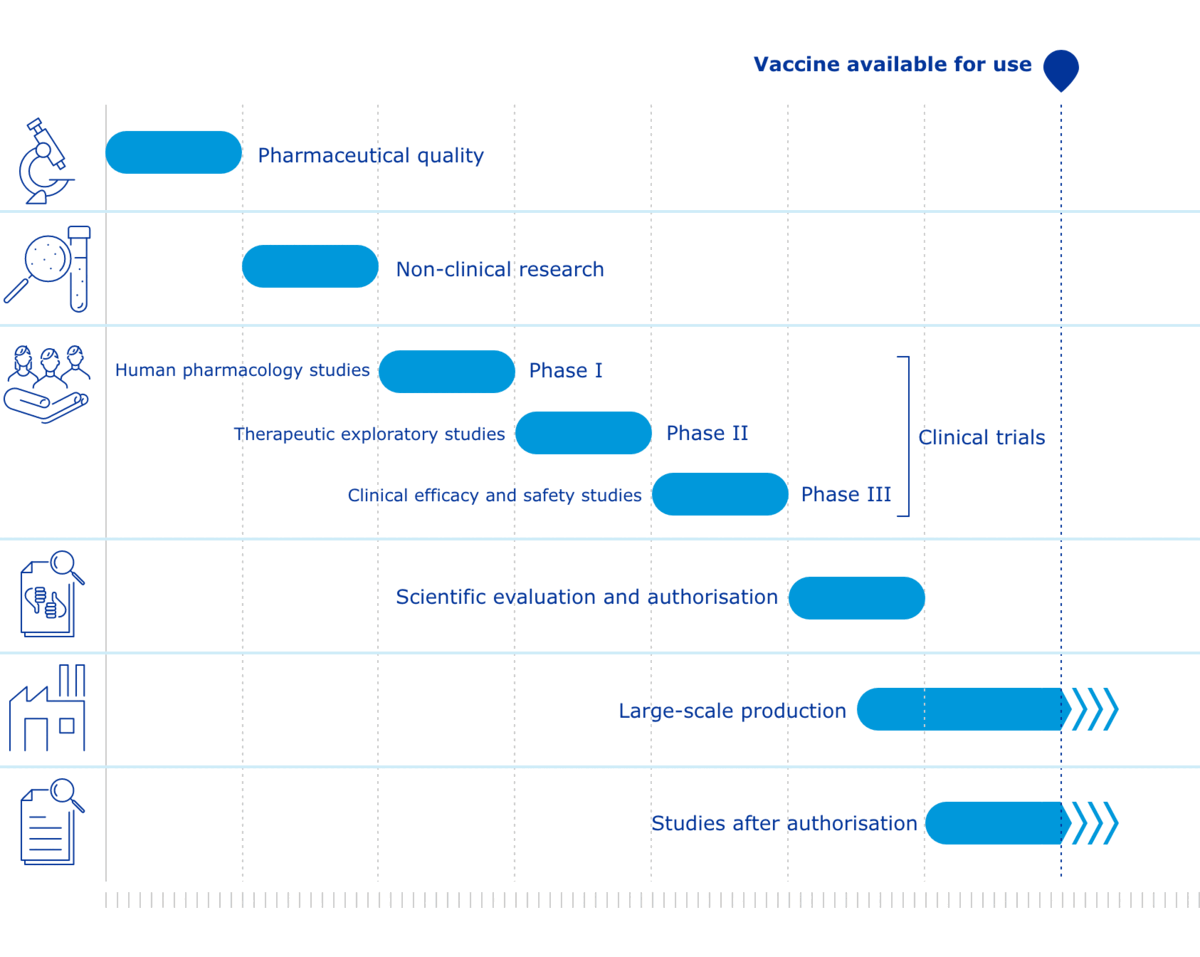
Standard Vaccine vs Covid Development Phases
Blue standard vaccine development phases and Covid in Red.
Source:
Red Biotech Market Size
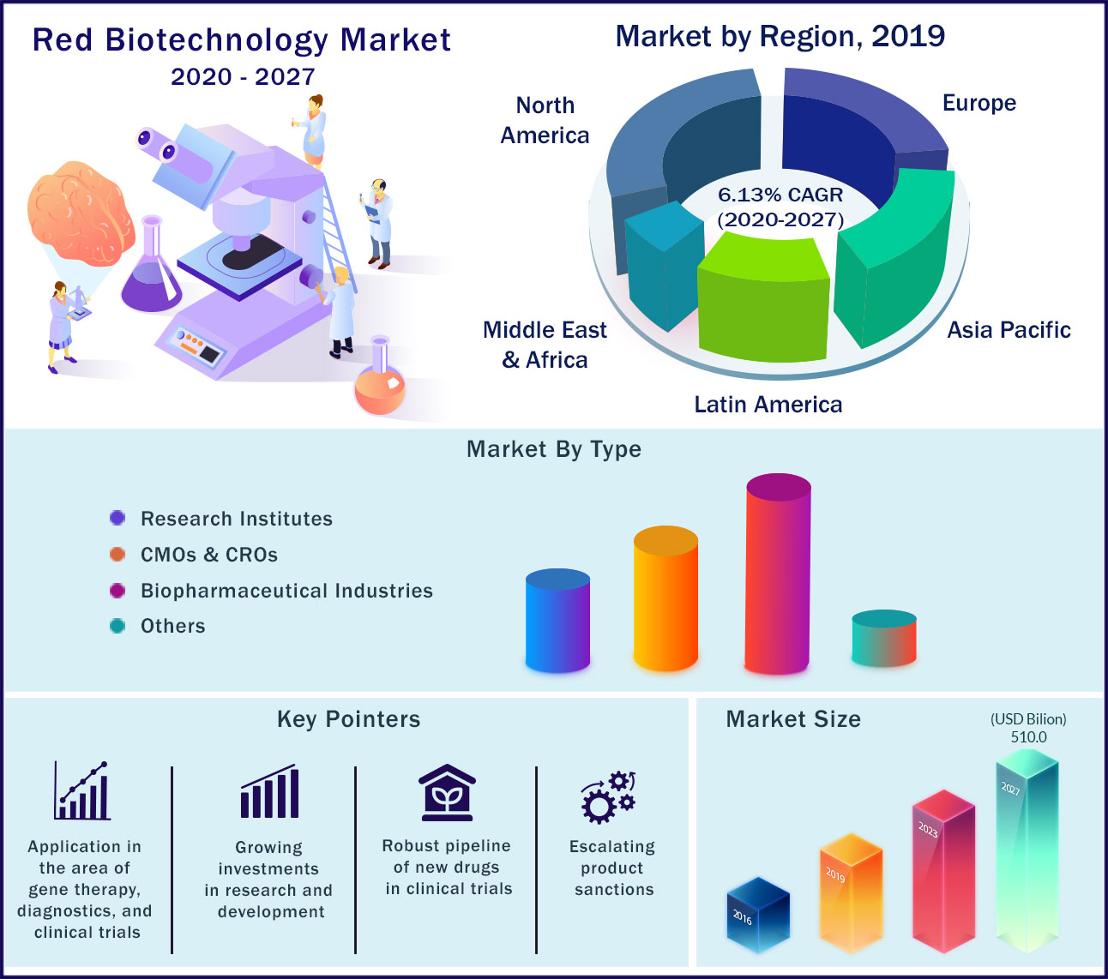
Red biotechnology market
The global red biotechnology market was valued at US$ 322 billion in 2020 and expected to reach US$ 510 billion by 2027, growing at a compound annual growth rate (CAGR) of around 6.13% during period 2020 to 2027.
Source
https://www.precedenceresearch.com/red-biotechnology-market
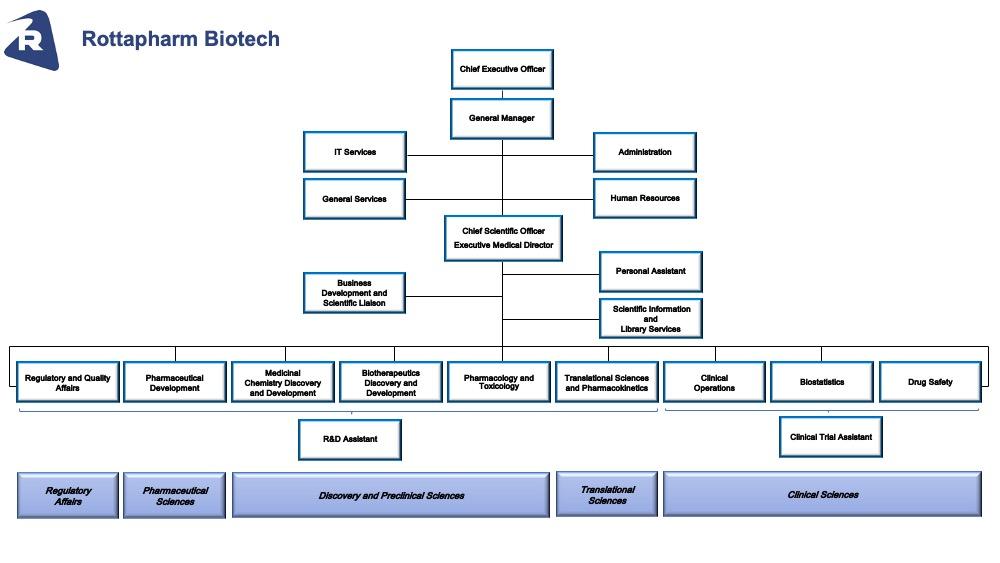
Red Biotech - Organization Structure
Organizations

efpia.eu
The European Federation of Pharmaceutical Industries and Associations (EFPIA) represents the biopharmaceutical industry operating in Europe. Through its direct membership of 36 national associations, 39 leading pharmaceutical companies and a growing number of small and medium-sized enterprises (SMEs), EFPIA’s mission is to create a collaborative environment that enables our members to innovate, discover, develop and deliver new therapies and vaccines for people across Europe, as well as contribute to the European economy.
Website NewsNews

BioPharma Drive
BioPharma Dive provides in-depth journalism and insight into the most impactful news and trends shaping biotech and pharma. The newsletter and website cover topics ranging from clinical readouts to FDA approvals, gene therapy to drug pricing and M&A to research partnerships.
Website
Labiotech.eu
Labiotech.eu is the leading digital media covering the European Biotech industry.
Over 150,000 monthly visitors use it to keep an eye on the business and innovations in biotechnology. Hope you’ll enjoy reading our stories!
YouTube
What is BioPharma?
A brief insight into the biopharmaceutical industry so as to entice the next generation of bright and caring people to get involved.
VideoA brief history of biopharmaceutical medicines
You may have heard of biopharmaceuticals - complex medicines made from living cells or organisms. Join Teva's Steffen Nock as he takes us on a brief journey through time that charts where biopharmaceuticals came from and where they might be going.
Pharmaceutical Product Life Cycle Management Strategies
The life cycle of pharmaceutical products is a bit different than consumer goods, this video explains the stages of the life cycle of a new drug, namely early, middle, and late stages and what are the strategies implemented by pharmaceutical companies to extend the life cycle over a longer period of time.
VideoMarketeers on Marketing: BioTech Marketing with Lisa Isailovic
NIA Creative interviews Lisa Isailovic, Director of Marketing for Aplegen, for the NIA Creative MediaCast Vlog Series "Marketeers on Marketing". In this episode, we focus on BioTech Marketing. Topics covered include the challenge of marketing products that are necessary in the lab, but are not necessarily "life-saving, as well as how to creatively market very young companies that literally don't have a brand much less a product
VideoPharmaceutical Sales Call - Best Practice Ideas, Verbiage Examples, Closing Tips, and more.
Pharmaceutical Sales Call Best Practices - you can use these tips create better interactions in your pharma and medical device sales calls that will result in more consistent sales.
VideoHow are medicines evaluated at the EMA.
Speaker: Nathalie Bere, EMA
Training session for patients and consumers involved in EMA activities
10 December 2013
VideoUnderstanding Pharmaceutical industry by Kris Kristensen.
Entering the world of pharmaceuticals can seem like going into a black hole. From the nuances of drug discovery to the complexity of governmental regulations, understanding how pharmaceutical companies operate seems almost incomprehensible.